Fig. 1, Artemisinin
This is a historical molecule. The first use of the tri-cyclic peroxy like (two oxygen atoms bonded to each other) structure was noted in 200 BC. Throughout the past 2000 years, Chinese herbalists used the plant from which this molecule derives, Artemisia Annua, for various maladies.
In the 1960s and 1970s the Chinese government established a program exploring the use of some 200 traditional herbs for the treatment of the growing threat of Malaria, a disease most commonly caused by an infection of a parasite called Plasmodium falciparum. Out of those trials, artemisinin was the only one that was turned into a drug.
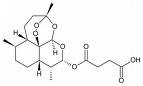
Artemisinin has an unusual structure for a drug like compound. The double oxygen peroxy group is not very stable. Indeed, the poor bioavalability has to be offset through combination therapies. Derivatives such as artesunate (below, note the extra 4 carbon tail) have also been used as alternative, longer lasting treatments.
As a side note, this 4 carbon tail is a functional motif found in some hdac inhibitors such as sodium phenylbutyrate and a recent breakthrough drug, vorinostat or SAHA. The side chain increases the hydrophybicity of the molecule and perhaps makes a better binding partner to cellular targets.


Fig. 3, Sodium Phenylbutyrate
 Fig. 4, Vorinostat - note the form of the carbon chain
Fig. 4, Vorinostat - note the form of the carbon chain
The mechanism of action for artemisinin is not clear, but it seems that the peroxide group is broken by free heme groups that are released by parasitic digestion of hemoglobin.
Recently, the HHV-6 Foundation, a not for profit organization exploring the role of the Human Herpes Virus 6 (HHV-6) in various diseases, has started funding a project to explore the use of artesunate for HHV-6 infection. It is very intriguing that a drug that has been used to disrupt a parasitic infection might also be effective against a viral infection.
There is much more to explore in a post about artemisinin. There are fears right now that monotherapy in third world countries, with artemisinin or its derivatives, is leading to resistance in Plasmodium falciparum. Separately, the synthesis of artemisinin is an important challenge as the extraction and growth of the plant source is impractical to treat the large number of people afflicted by malaria.
 Fig. 4, Vorinostat - note the form of the carbon chain
Fig. 4, Vorinostat - note the form of the carbon chainThe mechanism of action for artemisinin is not clear, but it seems that the peroxide group is broken by free heme groups that are released by parasitic digestion of hemoglobin.
Recently, the HHV-6 Foundation, a not for profit organization exploring the role of the Human Herpes Virus 6 (HHV-6) in various diseases, has started funding a project to explore the use of artesunate for HHV-6 infection. It is very intriguing that a drug that has been used to disrupt a parasitic infection might also be effective against a viral infection.
There is much more to explore in a post about artemisinin. There are fears right now that monotherapy in third world countries, with artemisinin or its derivatives, is leading to resistance in Plasmodium falciparum. Separately, the synthesis of artemisinin is an important challenge as the extraction and growth of the plant source is impractical to treat the large number of people afflicted by malaria.
A joke at every end:
What do you call a sheep with no legs?
A cloud.